Anewly approved pill to prevent HIV infection is creating hope for inroads in the global fight against AIDS, though questions about its cost, access and appropriate uses remain.
In July, the U.S. Food and Drug Administration approved a drug known as Truvada — the brand name for the medication emtricitabine/tenofovir disoproxil fumarate — for daily use as a pre-exposure prophylaxis, or PrEP, “in combination with safer sex practices to reduce the risk of sexually acquired HIV infection in adults at high risk,” according to an FDA statement. While the drug is not suitable for everyone, likely candidates include members of so-called “discordant” couples in which one person is HIV-positive.

Joe Clement, right, takes a rapid HIV test from a visitor at a San Francisco community health center in 2009. New tools are being added to the arsenal in the fight against HIV/AIDS, such as at-home HIV tests and a new prevention pill.
Photo by Justin Sullivan, courtesy Getty Images
“I’m optimistic that for some people it could be very beneficial,” APHA member Ken Mayer, MD, medical director at Fenway Health in Boston and visiting professor at Harvard Medical School, told The Nation’s Health. “I don’t think of PrEP as a panacea. It’s part of a package of approaches.”
While FDA’s approval of Truvuda as a pre-exposure tool is new, the drug itself is not. In 2004, the agency approved Truvada in combination with other drugs as an antiviral to treat those already infected with HIV.
Before the drug’s latest approval, two large clinical trials looked at medication safety and effectiveness in HIV prevention. In one study, Truvada was 42 percent effective in reducing the risk of HIV infection among HIV-negative men or transgender women who have sex with men who were determined to have high risk for HIV infection. In a study of heterosexual couples, use of Truvada reduced the risk of HIV infection by 75 percent. FDA officials said the eight-year lag between Truvada approval to treat HIV-positive people and approval as a prevention tool for those who are HIV negative was due to the need for clinical studies.
The drug carries side effects, and some studies have shown the medication could be linked to kidney and bone problems. Price is also an issue, as the drug may cost as much as $10,000–$13,000 a year.
“It is unclear yet how insurance companies will handle the newly approved on-label use of the drug,” APHA HIV/AIDS Section Chair Christopher Fisher, PhD, assistant professor of health promotion at the University of Nebraska Medical Center College of Public Health, told The Nation’s Health. “While PrEP may be a great new tool in the HIV prevention specialist’s toolkit, it will likely not be available to many of those who most need it, due to cost alone. Policymakers and drug company marketers will need to take costs of the drug into account when determining how best to implement PrEP as part of a multi-pronged approach to HIV prevention in the United States.”
FDA is requiring the medication to carry a boxed warning about prescribing Truvada only to those who are confirmed to be HIV-negative before taking the daily pill. The warning also recommends anyone taking Truvada as an HIV preventive to be tested for HIV at least every three months. The medication was approved for HIV prevention also with a risk evaluation mitigation strategy, or REMS, “to minimize the risk to uninfected individuals of acquiring HIV infection” and also to reduce the likelihood of the emergence of an antiviral-resistant strain of HIV.
“Education is the key,” Debra Birnkrant, MD, director of the Division of Antiviral Products in FDA’s Center for Drug Evaluation and Research, said during a July 16 news conference. “We are committed to working with our public health service colleagues and the HIV/AIDS community to develop guidelines and other resources on how to safely and effectively use Truvada for pre-exposure prophylaxis in combination with other HIV prevention methods so we can fully achieve the public health benefit Truvada for PrEP represents.”
The Centers for Disease Control and Prevention has issued draft guidelines for providers on prescribing Truvada for HIV prevention among men who have sex with men. The agency is also working with the U.S. Public Health Service on guidelines for using pre-exposure prophylaxis as an HIV prevention tool. World Health Organization guidelines are available as well.
As for concerns that the medication could encourage risky sexual behavior, FDA officials said that result was not apparent during the clinical trials.
“What we found was that in actuality, condom use increased over time,” Birnkrant said. “And sexually transmitted infections either remained as they were (at the) baseline or also decreased.”
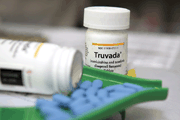
In July, FDA approved Truvada for preventing HIV infections.
Photo by Justin Sullivan, courtesy Getty Images
Studies of Truvada for HIV prevention showed a range for adherence to the recommended dose of one pill daily, Birnkrant said. In a study of men who have sex with men, compliance was about 30 percent, but in a study of heterosexual couples, 80 to 90 percent took the medication as directed.
“It’s hoped that with conversations between health care providers and individuals seeking the use of PrEP that adherence will be high,” Birnkrant said.
Mayer, who conducted research on the medication at a federally funded community health center in Boston, said he does not expect a rush of people clamoring for Truvada as an HIV prevention tool. Yet he sees approval of the drug as a pathway toward more prevention methods.
“It’s not a magic pill by itself,” Mayer said. “To me, one of the biggest challenges, and some of the work I’m doing with my colleagues is, how do we educate medical providers? How do you screen people who might benefit from this? How do you know who might be taking it responsibly?”
Several sessions at APHA’s 140th Annual Meeting in San Francisco in October will highlight pre-exposure prophylaxis-related research, said HIV/AIDS Section Program Planning Chair Jason Coleman, PhD, MSPH, assistant professor at the University of Nebraska at Omaha.
“We need to be sure to educate our populations about what PrEP is and what it does and what it does not do,” Coleman told The Nation’s Health. “It’s not an HIV vaccine. And I think we have to be careful to make sure people understand that it’s not an HIV vaccine.”
Moises Agosto of the National Minority AIDS Council called the FDA’s approval of Truvada for HIV prevention an “exciting new intervention” with “the potential to make significant inroads in the fight to bring an end to the HIV/AIDS epidemic.” The HIV Medicine Association warned the new approval “must not contribute to HIV-related health disparities,” said Association Chair Judith Aberg, MD, FIDSA, by diverting funds from safety-net programs that serve people with HIV. She also pressed for education for health care providers and further study of pre-exposure prophylaxis among women, especially minority women.
Earlier in July, FDA approved the OraQuick In-Home HIV Test as an over-the-counter home test for the virus. One of the barriers in the AIDS fight is that one in five of the about 1.2 million Americans with HIV do not know they are infected. One key is funding for scientific advances against HIV/AIDS, a sentiment that was shared during the XIX International AIDS conference in Washington, D.C., in July.
“Continue to invest in science,” said Diane Havlir, MD, co-chair of the conference and professor of medicine at the University of California, San Francisco. “Scientifically, when it comes to AIDS, there is more light at the end of the tunnel than there has ever been in the three decades of the epidemic.”
For more information, visit www.fda.gov. For more HIV/AIDS news, see Page 31 of this issue.
- Copyright The Nation’s Health, American Public Health Association