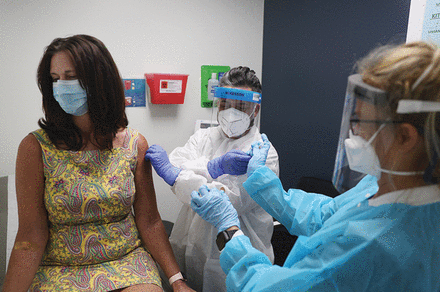
Volunteer Lisa Taylor, left, receives a vaccination from Jose Muniz, RN, center, during a COVID-19 vaccine trial at Research Centers of America in Hollywood, Florida, in August. More than 165 vaccines were under development this summer.
Photo by Craig Walker, courtesy The Boston Globe/Getty Images
After a vaccine for COVID-19 is developed, clinically tested, approved and manufactured, the process of administering it is expected to be the biggest immunization campaign in U.S. history.
“Everyone is susceptible, and so we need to be working to vaccinate the entire population,” said Kris Ehresmann, MPH, RN, director of the Infectious Disease Epidemiology, Prevention and Control Division at the Minnesota Department of Health. “It’s a huge undertaking.”
With more than 165 vaccines against COVID-19 in development around the world and over two dozen in human trials, Ehresmann and her team are leading Minnesota’s planning efforts for rolling out, distributing and administering the eventual vaccine — or vaccines — that make it to market.
Like most health departments, the Minnesota agency has trained for years on deploying mass vaccinations in the midst of a pandemic, and its immunization staff are specifically skilled in managing large vaccination programs, data systems and public education campaigns. The department is also drawing on its experience during the H1N1 pandemic, when nationwide public health efforts immunized about a quarter of the country over the course of the 2009-2010 flu season.
“It’s not enough to have a clinically successful vaccine, you also have to have a socially acceptable one.”
— Monica Schoch-Spana
Those lessons will help, but COVID-19 — and the chaotic, under-resourced federal response to stop it — puts the coming vaccine challenge in a category all its own. Like H1N1, for example, Ehresmann is preparing for high vaccine demand, initial limited doses and supply chain problems. But unlike H1N1, which is seasonal, there will be no break: “There’ll be a huge demand, and it won’t go away,” she said.
Health officials are also preparing for some COVID-19 vaccines to require two doses to be effective, which represents a big challenge for tracking and notification systems and making sure people stick with the same vaccine product for each dose.

1 in 3 people would not get a COVID-19 vaccination if a free, FDA-approved option was available, a July 20-Aug. 2 Gallup poll found. Vaccine hesitancy is one of the issues officials are preparing for.
Image by Aaron Warnick
New vaccine providers around Minnesota need to be integrated into state and local immunization systems to track needs and supplies as well as immunization rates and gaps. Providers need to be educated on safely storing the millions of doses that will eventually get delivered at very specific temperatures so they remain potent. Then there is educating the public to build trust and ensuring limited supplies are distributed first to those at greatest risk.
Keeping an equity lens front and center will be key to those efforts, Ehresmann said, not only for creating a framework for prioritizing and distributing initial doses, but in building trust and addressing vaccine hesitancy in communities historically marginalized and excluded by health systems. Recent surveys, for example, find Black Americans, who suffer a disproportionate share of COVID-19 deaths, are much less likely to say they are willing to get a coronavirus vaccine than Hispanics and whites.
“Vaccine hesitancy is a huge concern for us across the board,” said Ehresmann, noting that just a few years ago, Minnesota experienced the largest measles outbreak it had faced in decades. “So when we’re planning for a COVID vaccine, we need to be really thoughtful about the rollout — we want to make sure our handling of this process doesn’t exacerbate existing vaccine hesitancy.”
Of course, no one knows exactly when a COVID-19 vaccine will be available, though White House officials are promising one at record speed, which has its own messaging problems when it comes to building confidence in the vaccine’s safety. When one is available — which could happen while the U.S. outbreak is still largely uncontrolled — state and local public health will know what to do. The real question is whether leaders will listen, let them lead and give them the resources to succeed.
“Public health really needs to be in the lead,” Ehresmann said. “That’s not to say we can’t benefit from input and insights from other sectors. But I do think it’s important to recognize what public health brings to the table.”
Communication, capacity challenges
By January, Operation Warp Speed, the federal government’s COVID-19 vaccine plan, aims to deliver 300 million vaccine doses. As of August, however, state and local health departments — the backbone of the nation’s immunization system — were still waiting on key details about a national distribution plan.
Preparations, however, were still well underway, with the assumption that federal, state and local health agencies will work in concert to coordinate vaccine distribution and prioritization, building off existing immunization programs such as Vaccines for Children, which already distributes millions of doses a year. On Aug. 4, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, wrote to state immunization and preparedness officials on “interim assumptions and recommended action steps” for vaccine planning.
In the letter, Messonnier said initial groups recommended for COVID-19 vaccination will likely be essential workers as well as staff and residents in long-term care facilities. She said to expect COVID-19 vaccine distribution to be managed centrally, with doses to be sent directly to providers, such as doctor’s offices and pharmacies. She recommended onboarding new providers into immunization systems, reaching out to vulnerable communities and partnering with trusted messengers.
Messonnier also announced a new COVID-19 vaccine planning pilot in five jurisdictions: California, Florida, Minnesota, North Dakota and Philadelphia. Pilot participants will work with staff at CDC and the Department of Defense. Two committees at the federal level — CDC’s Advisory Committee on Immunization Practices and another convened by the National Academy of Medicine and National Academies of Sciences, Engineering and Medicine — are expected to release recommendations on equitable vaccine allocation and prioritization this fall.
Jim Blumenstock, MS, senior vice president for pandemic response and recovery at the Association of State and Territorial Health Officials, said the lack of firm specifics at the national level has not stopped preparations at the state and local levels and “nobody is standing around and waiting.”
But state health officials need to coordinate with their national counterparts, and Blumenstock said those lines of communication had been “very limited” until mid-August, when CDC started having weekly vaccine planning calls with state officials.
State health departments are prepared for a widespread vaccination campaign, he said, with the caveat that agencies have been under-resourced and overtaxed for years. ASTHO is calling for billions in additional emergency funds to support a COVID-19 vaccine campaign and scale up existing immunization systems and capacity.
“We’ve overcome that communication gap, if you will, and now we are moving full steam ahead,” said Blumenstock, an APHA member.
Claire Hannan, MPH, executive director of the Association of Immunization Managers, said a key focus of current planning efforts is identifying high-risk groups — such as essential workers and front-line health workers — and making plans in concert with local providers and trusted community leaders to get them immunized. Another big focus is making sure existing immunization systems are ready to take in huge flows of new data from new providers and track the data according to critical new indicators, such as occupation.
Typically, such as with the Vaccines for Children program, local immunization staff will conduct on-site visits to ensure vaccinators are safely storing and administering vaccines. Hannan, an APHA member, said that will not likely happen for COVID-19 — “We just don’t have the resources for that” — which is why it is so important to build on existing networks and infrastructure.
“Emergency funding will be critical,” she told The Nation’s Health. “Health departments are stretched very thin, immunization staff have been shifted to COVID-19 response, and that’s likely to continue even when we do have a vaccine.”
But of all the vaccine challenges ahead, Hannan said public acceptance of a COVID-19 vaccine might give her the most anxiety. According to a Gallup poll released in August, only 65% of Americans said they would get a free, FDA-approved vaccine if it were ready that day. Hesitancy is especially high among Black Americans: Recent survey results from the Pew Research Center found 54% of Black adults said they would definitely or probably get a COVID-19 vaccine, compared to 74% of both Hispanic and white adults.
“There’s a history of experimentation on Black men and women, so there’s a profound sense of distrust there,” said Monica Schoch-Spana, PhD, a senior scholar at the Johns Hopkins Center for Health Security. “It’s not enough to have a clinically successful vaccine, you also have to have a socially acceptable one...We can’t underestimate the importance of working with community partners, especially those coming from a civil rights perspective.”
Schoch-Spana co-authored a July report on public health’s role in COVID-19 vaccine planning. The report addresses big challenges — such as allocation, prioritization, distribution, communication and engagement — and calls for an overarching philosophy that puts people at the center in every step of the vaccine endeavor. The U.S. is especially behind on vaccine communication and engagement, she said.

In March, research assistant Noe Mercado prepares a test at Boston’s Barouch Laboratory, which is working on a COVID-19 vaccine using the virus’ genetic sequence. Public health officials are making plans to deploy a vaccine once it is approved.
Photo by Joe Raedle, courtesy Getty Images
Vaccine education has gotten increasingly difficult and complex with the rise of the anti-vaccination movement and social media platforms that make it easy to quickly spread misinformation. And the ongoing politicization of the pandemic will make things even harder. But Schoch-Spana, an APHA member, said there are effective counter tactics, such as sticking to personally relevant and meaningful narratives about the benefits of vaccination.
“There’s no single answer for the misinformation problem,” she told The Nation’s Health. “But the strongest solution is to put out a steady stream of comprehensible, personal, good information in its place and crowd it out.”
Planning is also underway at local health departments, which play a crucial role in estimating local vaccine needs, working with local providers, identifying vulnerable residents and administering vaccines — a key safety net function, said APHA member Oscar Alleyne, DrPH, MPH, chief of programs and services at the National Association of County and City Health Officials. At the same time, he said, vaccine capacity inside local agencies is depleted, with all hands needed to chase and contain the virus outbreak.
“Every local health department will tell you, ‘We’ve been planning, training for and exercising mass vaccinations for years — we’re ready,’” Alleyne said. “But we need another round of emergency funding and some accountability about how those funds are getting down to the local levels and reaching those who need it.”
For more infomation on COVID-19 vaccine development, visit https://covid-19tracker.milkeninstitute.org.
- Copyright The Nation’s Health, American Public Health Association